An In-Depth Exploration of Neurovascular Coupling
Discovering the Complex Mechanisms Behind the Correlation Between Neural Activation and Blood Flow in the Brain
whats covered in this issue
- Assess the current knowledge of the NVU → specifically focused on one of its prototypical functions: the coupling between neural activity and CBF
- Review observations related to the contribution of different NVU cells to neurovascular coupling
- An attempt to provide a comprehensive picture of how activation of restricted groups of neurons is able to generate hemodynamic responses engaging the entire cerebrovascular tree, from capillaries (deep in the brain) to the pial arteries (brain surface)
the brains design glitch
Neural Activation is accompanied by a complex sequence of cellular, metabolic (get or make more energy), and vascular (vessels that carry blood) mechanisms.
Our brains, an organ of significant sophistications consumes a large amount of energy, 20% of the body’s energy to be exact. The problem is that it does not contain any energy storage aka there is no reservoir in our brain particularly to store fuel for use when needed.
As such, the brain requires a constant input of oxygen and glucose through our blood supply. Considering the dynamic and regionally diverse energy requirements imposed by neuronal activity, blood flow needs to reach the brain at the tight time and place and in the right time.
the neurovascular unit (nvu)
There has been a long-standing interest in how exactly the brain regulates its own blood supply, driven by the early realization that regional changes in cerebral blood flow (CBF) may provide a window on brain function. The large body of work spanning over almost two centuries revealed a remarkable complexity in the interaction between the brain and its vessels that is unmatched by the vasculature in other organs.
In 2001, the concept of the neurovascular unit (NVU) was emerged to emphasize the unique relationship between the brain cells and the cerebral vasculature.
A large assumption that was circling around at the time was the idea that the brain cells and cerebral blood vessels were completely different entities and because of that, neurons had little to do with the vasculature and vice versa.
The idea of the NVU absolutely trashed this assumption and stressed the symbiotic relationship between brain cells and cerebral blood vessels.
structural diversity of the nvu along the cerebrovascular tree
The association between neurons and vessels vary significantly across the cerebrovacular network.
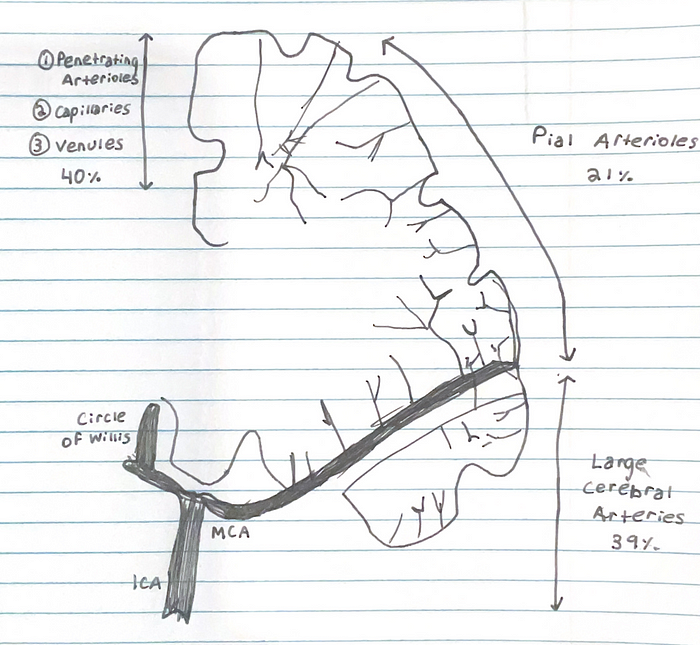
1) Starting off at the the Circle of Willis (the joining area of several arteries at the bottom side of the brain), cerebral vessels run on the brain surface within the subarachnoid space (pial arteries and arterioles), forming a highly collateralized network
- Subarachnoid Space → The subarachnoid space is a CSF-filled compartment within which are the major cerebral blood vessels.
2) Pial arterioles have multiple layers of smooth muscle cells (SMCs) separated from the endothelium by a prominent elastic lamina
- Smooth Muscle Cells → The prime function of the arterial smooth muscle cell (SMC) in individuals is to contract and relax, thereby regulating blood flow to target tissues
- Endothelium → A thin membrane that lines the inside of blood vessels. It represents an active interface between blood and central nervous system
- Elastic Lamina → Provide elasticity and prevent the vascular wall from collapsing
3) Although not in direct contact with brain cells, pial vessels are richly innervated (supply nerves to) by nerve fibers originating from peripheral autonomic and sensory ganglia, including the superior cervical, sphenopalatine, and trigeminal ganglia. These nerve fibers, which express a wide variety of neurotransmitters and neuropeptides, run at the adventitial-media border, forming an intricate mesh enveloping/surrounding the vessels
- Ganglion → A collection of neuronal bodies found in the voluntary and autonomic branches of the peripheral nervous system (nerve cell bodies that is not contained in the CNS). In contrast, cluster of neuronal bodies in the CNS are nucleus. Ganglia can be thought of as a synaptic relay stations between neurons. The information enters the ganglia, excites the neuron in the ganglia and then exits.
- Sensory Ganglia → Cell bodies of sensory neurons
- Autonomic ganglia → Cell bodies of efferent neurons from the autonomic nervous system.
- Adventita → The outer layer of connective tissue that surrounds an artery
- Media → The tunica media , or media for short, is the middle tunica (layer) of an artery or vein. It lies between the tunica intima on the inside and the tunica Adventita on the outside
4) Pial arteries dive into the substance of the brain surrounded by an extension of the subarchnoid space, the perivascular space, a virtual space delimited by the vascular basement membrane and the glia limitans
- Perivascular Space → Fluid filled space surrounding certain blood vessels
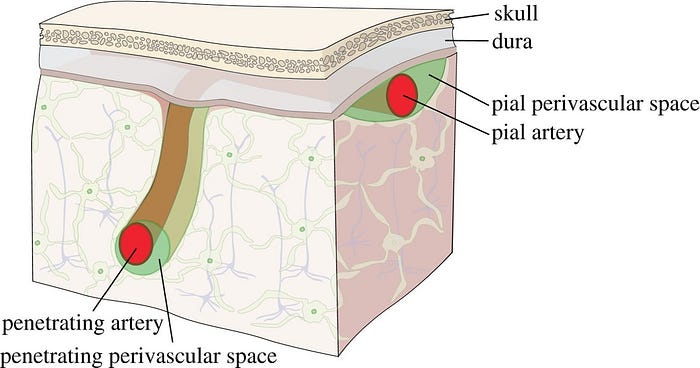
- Vascular Basement Membrane → Separates the endothelial cells from neurons and glial cells and contributes to vessel development and formation and maintenance of the blood-brain barrier
- Glia Limitans → The outermost layer of nervous tissue of the brain. Main role of the glia limitans is to act a physical barrier against unwanted cells or molecules attempting to enter the CNS
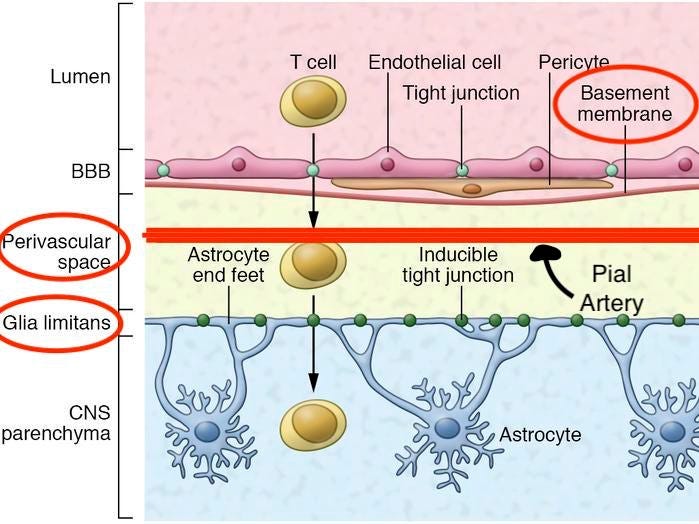
5) Penetrating arterioles are endowed with a thinner SMC layer that becomes a single layer. At this layer, perivascular nerves are more spaced, and the elastic lamina becomes less prominent. The perivascular compartment contains several cell types, including perivascular macrophages(PVMs), Mato cells, collagen fibers
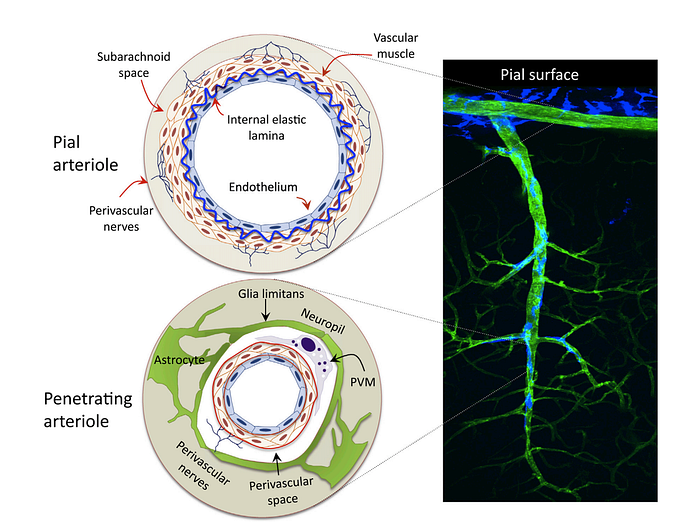
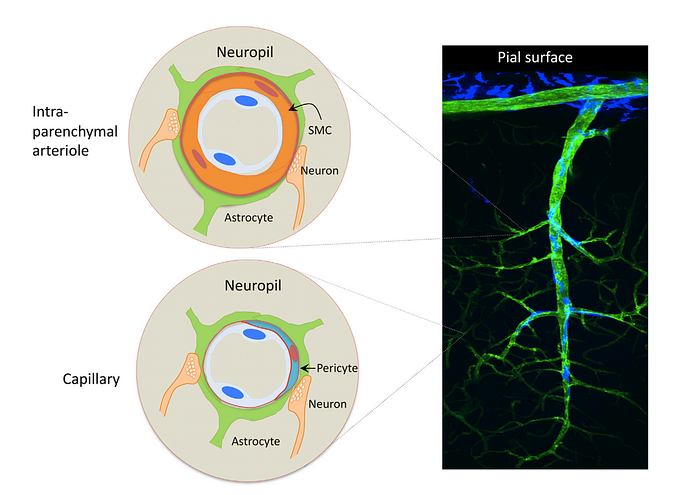
6) As arterioles pentrate deeper into the brain (intraparenchymal arterioles), the glial membrane and the vascular basement membrane fuse together, obliterating the perivascular space (due to the “fused gliovascular membrane”). At this level, arterioles have a single or discontinuous layer of SMCs, lack perivascular nerves, and are encased in astrocytic endfeet. Occasionally, axonal terminals or dendrites are seen in close apposition to the vascular basement membrane, often with an intervening glial leaflet. These neural processes originate from interneurons or from subcortical and brain stem nuclei projecting to the cortex, such as the basal forebrain cholinergenic nuclei, locus coeruleus, and raphe magnus
7) Endothelial cells extend protrusions through the basal lamina into SMCs (myoendothelial projection) enriched with gap junctions
8) As arterioles transition into cerebral capillaries, SMCs are replaced by pericytes, mural cells embedded into the endothelial basement membrane, covering approximately 30% of the vascular surface and occasionally extending “peg and socket” protrusions into endothelial cells. Like in arterioles, the circumferences of the capillaries is covered by astrocytic endfeet, although neural processes can be at times observed next to the capillary basal lamina
neurovascular coupling
As said above, one of the most appreciated concepts related to the NVU is the link between neural activity and CBF.
In the resting brain, CBF varies in proportion to the energy consumption of each brain region. Thus, flow is higher in regions with higher energy utilization (e.g. the inferior colliculus) and lower in regions with lower energy use, like the white matter.
Furthermore, increases in neural activity lead to increases in CBF — highly restricted to the activated areas (functional hyperemia )
The spatial and temporal correspondence between neural activity and the associated hemodynamic response is sufficiently precise, at least at the regional level, that the flow response can be used to map brain function (functional brain imaging). However, at the microvascular level, the CBF increase in some regions exceeds the area of activation.
For instance, in the auditory, visual, and cerebellar cortex, the vascular response does not faithfully match the activated area, whereas in the olfactory bulb, there is close overlap between the two.
Non-overlapping vascular and neuronal topology in the neocortex and restrograde vasodilation are likely responsible for such lack of fidelity. Because hemodynamic signals are the most powerful tool at our disposal for functional brain mapping, their spatial alignment with neural activity is becoming increasingly relevant as functional imaging increases.
The close relationship between neuronal and vascular function is also illustrated by the changes in neurovascular coupling that takes place in a brain that is developing.
In rodents before post-natal day 11, neural activation is not associated with sustained CBF increases, leading to an absent or negative BOLD signal (decrease of blood flow would produce less oxygen supply to the area and hence a negative BOLD signal). The lack of a flow response may be important for vascular development because the resulting hypoxia is a critical stimulus for cerebral angiogenesis.
However, in the second and third week of life, neural activity leads to increasingly larger BOLD signals. This transition between the second and third post-natal week corresponds to dramatic neurovascular and systemic changes, including increases in:
- vascular density
- synaptogenesis — explosion of synapse formation between neurons during early brain development
- myelination — increase in the fatty sheath surrounding neuronal processes and fibers that increases the efficiency of electrical transmission
- connectivity
- energy metabolism
- resting CBF
- sensitivity of the cerebral microcirculation to vasoactive stimuli
- systemic blood vessel
Regardless of the fact that the factors responsible for the developmental shift in neurovascular coupling remain to be explained, these findings speak to the influence of key structural, metabolic, and functional changes in the developing brain on shaping cerebral perfusion (the amount of pressure needed to maintain blood flow to the brain).
feedforward vs. feedback mechanism?
Alterations in neural activity and metabolism are correlated with changes in CBF, however, the mechanisms linking these processes are a matter of debate.
The coupling between neural activity and CBF is thought to reflect the lack of energy reserves in the brain requiring a well-timed delivery of both oxygen and glucose restricted to the activated areas.
The flow increase may also be needed to clear the brain of potentially toxic by-products of brain activity (for instance, lactate, CO2, the the amyloid-b peptide [Ab], and tau as well as for brain temperature regulation.
These considerations have led to people thinking that blood flow is controlled directly by energy demand. In fact, this idea was originally proposed over a century ago by Roy and Sherrington (1890): “…the brain possesses an intrinsic mechanism by which its vascular supply can be varied locally in correspondence with local variations of functional activity.” In this view, regional blood flow is controlled by feedback mechanisms that are sensitive to variations in the concentrations of ionic and molecular metabolic by-products. These by-products, such as K+, nitric oxide (NO), adenosine, carbon dioxide (CO2) and arachidonic acid metabolites, may directly or indirectly alter blood flow by depolarizing (or hyperpolarizing) the vascular smooth muscle cells which trigger vasodilation (or vasoconstriction). Although intuitive, the idea that energy supply (i.e., local blood flow) is controlled directly by energy demand (i.e., metabolic activity) appears to be oversimplified.
An alternative possibility is that local blood flow is controlled directly by a feedforward mechanism involving neuronal signaling via neurotransmitters. Evidence for this mechanism suggests that astrocytes may play an important role in linking neurotransmitter activity to vascular responses. Astrocytes are a critical component for glutamate recycling. A cascade of chemical events within the astrocyte may then link the rate of glutamate cycling to the production of vasoactive chemical agents. In this view, neurovascular coupling is mediated by neuronal signaling mechanisms via glial pathways, rather than by mechanisms that sense energy consumption.
In addition, neurovascular coupling might be mediated by diffusion of products of neuronal activity without the involvement of glial cells. Finally, there is evidence that direct neuronal innervation of smooth muscle cells can also control blood flow.
Ultimately, it is likely that multiple mechanisms, both feedforward and feedback, function to mediate neurovascular coupling. It is important to note that neurovascular coupling must provide for continuous brain function which requires metabolic nutrients and the elimination of waste products such as CO2 and excessive heat.
Potential Feedforward and Feedback Mechanism Driving the Local Vascular Response Evoked by Synaptic Activity

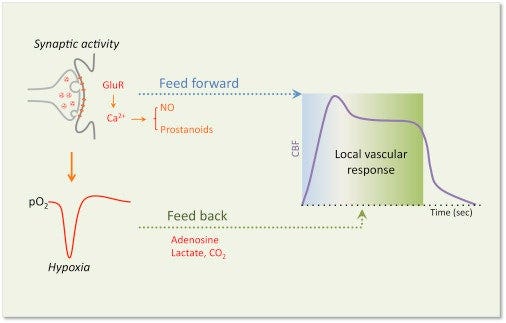
- Glutamate released by synaptic activates post-synaptic glutamate receptors (GluRs), leading to activation of Ca2+-dependent signaling pathways, resulting in the release of vasoactive factors that may drive the initial feedforward component (metabolism-independent) of the local vascular response in arterioles and capillaries
- At the same time, a reduction in tissue O2 caused by the increased energy consumption induced by activation leads to the accumulation of vasoactive by-products that may drive a secondary feedback component (metabolism-dependant) to better match the flow response to the metabolic needs of the tissue.
cellular bases of neurovascular coupling
In the past decade, there has been a lot of focus on the mediators responsible for nvc. This work suggested that multiple agents released by neural activity are involved.
However, it was also recognized that local release of vasoactive mediators could not account for hemodynamic changes in upstream vascular segments remote from the activated site. The availability of various tools to investigate the neurovascular interactions at the cellular level have provided greater insight into the cellular processes initiating, transmitting, propagating and implementing the vascular response.
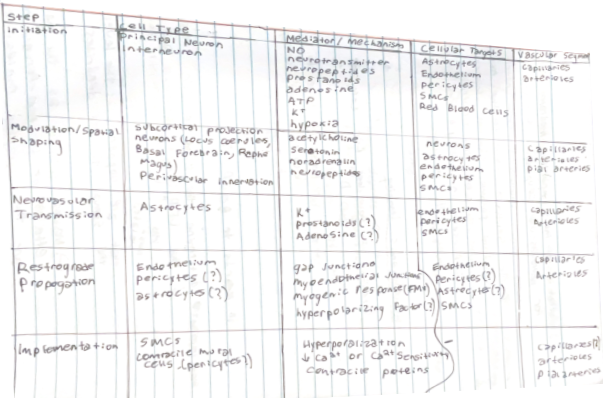
role of neurons in nvc — initiation of the local vascular response
TLDR.
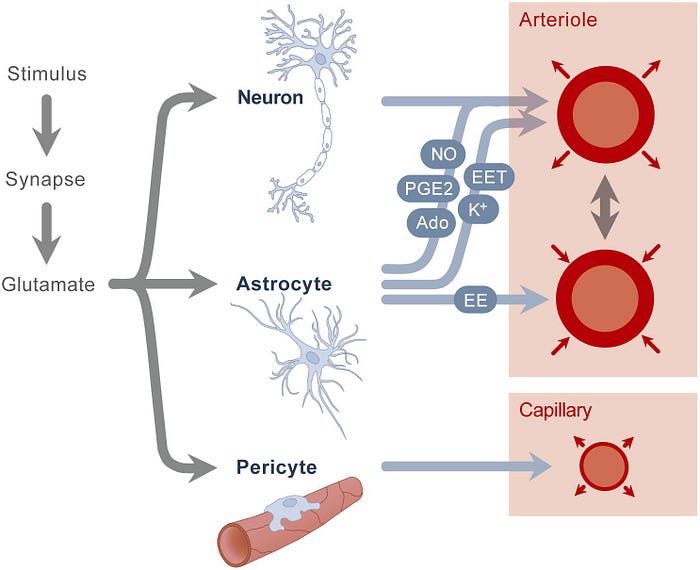
- One of the main processes involved in neurovascular coupling is done in glutamate
- So when we have a glutamate signaling then the glutamate receptor (glutamate receptor located primarily on the membranes of neuronal + glial cells) activation then the glutamate receptor activation leads to the activation of nitric oxide synthase and cox -2 which then leads to the production of vasodilators like nitric oxide and prostanoids (family of molecules that include prostaglandins (example)) → these lead to the increase in the diameter of blood vessels
- Interneurons are one of the most important neuronal types to determine the neurovascular types to determine the neurovascular coupling response
- So we know that certain interneurons do contain nitric oxide synthase and therefore lead to nitric oxide production which is one of the most potent vasodilators and then interneurons can also produce neuropeptide y (vasoconstrictor)
- Ionic changes role in determining the vasodilation and vasoconstrictor of arterioles to changes in extracellular potassium up to 12 millimolar leading to SMC relaxation (Changes in ions outside the cell to create the neurovascular coupling response)
in-depth analysis.
Neurons regulate CBF by generating signals that, either directly or though interposed cells, act on local blood vessels to initiate the vascular response.
Glutamatergic synaptic activity activates post-synaptic N-methyl-D-asparate (NMDA) and a-amino-3-hydroxy-5-methyl-4 isoxazol epropionic acid receptors, leasing to increases in intracellular Ca2+ and activation of Ca2+-dependant enzymes such as neuronal NO synthase (nNOS) and cyclooxygenase 2 (COX-2), which produce potent vasodilators (NO and prostanoid, respectively)
At the same time, glutamate acts on metabotropic glutamate receptors in astrocytes, triggering Ca2+ increases in these cells and production of vasoactive agents.
Adenosine, a potent vasodilator produced from ADP by ecto-nycleotidases, also contributes to the increase in CBF
In addition, a role for ATP acting on purinergenic receptors has been postulated both in the retina and neocortex
Finally, ionic changes evoked by neural activity, in particular the increase in extracellular K+. a vasodilator a concentrations of less than 12 mM, could also participate.
role of astrocytes in nvc
TLDR.
- Role to play in neurovascular coupling and a lot of signaling mechanism to determine the shape of the response
- Astrocytes produce a variety of factors that are both vasodilators + vasoconstrictors — together, they can determine the shape of the response
- Same glutamate released by neurons can (in astrocytes) cause a release of intracellular calcium leading to the activation of phosphilate A2 leading to the release of arachidonic acid which can be broken down into multiple molecules that have vasoactive properties — most important = Prostaglandin E2 and Epoxyeicosatrienoic acids (EETs)
- In astrocytes, theres a role for a calcium (Ca2+) mediated release of potassium (K+) — vascular SMC relaxation → “Minor Contribution”
- ATP leads to constriction (direct) or vasodilation (indirect)
role of endothelial cells in nvc
Endothelial Cells —
- Location: surround all vessels but mostly are at the level of capillaries
- Contain several vasodilators including no prostanoid, endothelin…
- Important for retrograde propagation to pial arterioles
- Released by a postsynaptic dendrite or cell body, and travels backwards across a chemical synapse to bind to the axon terminal of a presynaptic neuron
- Capillaries sending signals which are mostly electrical through gap junctions, via Ca2+ and K+ CAUSING retrograde propagation of the signal for vasodilation
role of pericytes in nvc
- Contractile cells that are contractile in nature so can change capillary diameter
- Mediated through Prostaglandin E2 + Nitric Oxide
Other Capillary Hypotheses
- Capillary Hypoxia (tissues starved of oxygen) increases deformability of red blood cells
- Decreased viscosity + Flow without a diameter change
- Increase in flow state due to decrease in velocity can increase shear stress causing endothelial release of visoactive factors
- Then lead to SMC and increase in diameter
- Parallel capillaries have different transit times and that limits the exchange of oxygen
- If we homogene transit time + increase flow vasodilation would occur
Main Role of Capillaries → Deliver Oxygen in blood to tissues
Key Cellular Steps Underlying Neurovascular Coupling

- Different type of cells and molecules play a role in diverse range of parts in the hemodynamic response
- So MANY effectors + cell types are important
- Need something that us a feed-forward mechanism that increases blood flow maintains the increased blood flow and then return to baseline → orchestration of this very complex multicellular response
What is the most important mediator. molecules for neurovascular coupling?
- Meta Analysis uncovered that nitric oxide synthase would be the most important pathway through which neurovascular coupling occurred. Present in multiple cell types that NVC occurs
Hemodynamic Imaging
How does fUS compare to BOLD?
- Most important to determine the kind of NVC response is the LFP (sub threshold activity from synaptic activity)
- Happening at the level of the soma and the dendrites
- Makes sense as the main effectors of the feed forward response are the synaptic molecules
Things that Have Screwed up BOLD
- Neg. BOLD has a more uniform relationship with electrophysiological component than positive BOLD
- Cannot interpret neg + pos the same
- NVC depends on the brain states
- Reduced hemodynamics in slow states and more activated in activated states
Thanks for reading! Connect on LinkedIn or feel free to email at mikaelhaji@gmail.com.
